Business Model
Selmod focuses on developing new anti-infective therapies. We are performing the preclinical research and clinical development of new disruptive antibiotics to the end-of-through phase 2 approval clinical studies.
Successful, ‘de-risked’ clinical candidates including all assets, projects or business units will be divested to existing partners.
Selmod has build-up a suitable research portfolio through internal scientific efforts, research collaborations with external public or private institutions, and in-licensing. We continuously explore new opportunities fitting to our business approach.
A core team of employees manages, coordinates and validates significant out-sourced activities.
Antimicrobial resistance therapies are an emerging market.
Currently more than 750’000 bacterial infections occur per year in the Europe and North America resistant to available therapies , causing over 40’000 deaths.
Over 1 million fungal infections - mainly in chronic sick patients -– are also reported every year. Over 30% of these are facing significant resistance formation.
Available antibiotics for the therapy of bacterial and fungal infections are getting more and more ineffective. Resistance formation, genetic exchange of the resistance between microbiological species and spread through our food stock and environment are major reason for the evolving antibiotic crisis.
The post-antibiotic era has become reality. The changing reimbursement options generate opportunities for our start-up.
At Selmod we inventing and developing disruptive antibiotics against new targets focusing on new structural types of chemical modalities. Thus, we overcome existing resitances and we provide life-saving therapies for our patients.
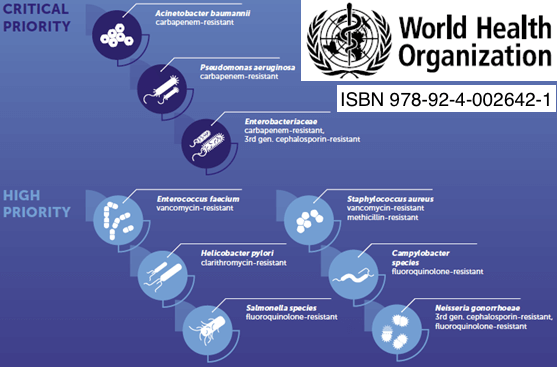
Priority pathogens for which new therapies are needed.
Selmod addresses the major health threats related to antimicrobial resistance.
Multi-drug resistant bacteria
Carbapenem resistant Enterobacteriaceae Enterobacterales ESBL-producing Enterobacteriaceae
Multidrug-resistant Pseudomonas aeruginosa
Carbapenem-resistant Acinetobacter baumannii
Life-threatening Fungi
Drug-resistant Candida spp., including Candida auris
Drug-resistant Aspergillus spp.
https://apps.who.int/iris/handle/10665/311820